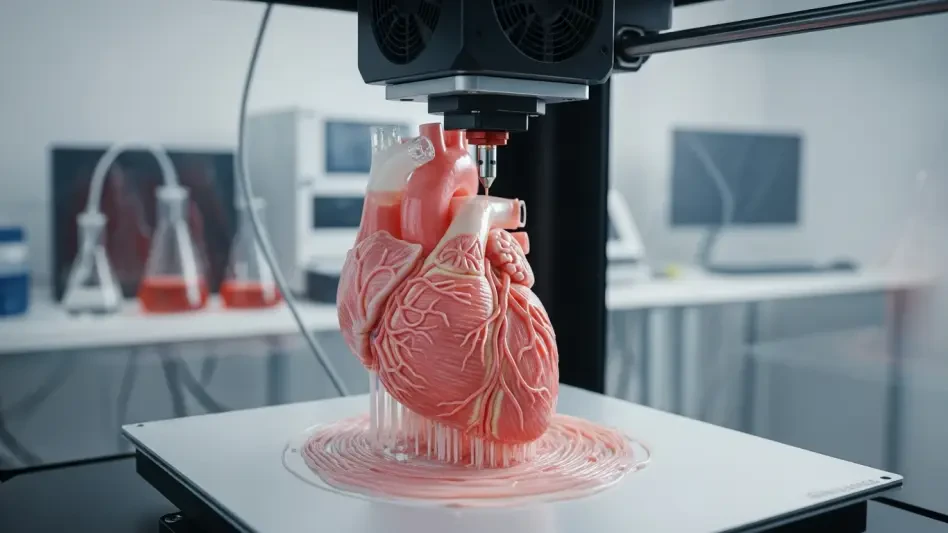
In the realm of modern medicine, a staggering challenge persists: the urgent need for precise models to study complex diseases like breast cancer, where traditional methods often fall short in replicating human tissue dynamics. Over 2.3 million new breast cancer cases are diagnosed globally each year, yet the tools to understand tumor behavior and test therapies remain limited by oversimplified cell cultures or inconsistent animal studies. Enter 3D bioprinting—a groundbreaking technology that constructs tissue-like structures layer by layer, offering a glimpse into a future where personalized treatments and organ regeneration could become commonplace. This review delves into the core of this innovation, exploring its mechanisms, applications, and potential to transform healthcare.
Core Principles and Emergence of the Technology
At its heart, 3D bioprinting involves the precise deposition of bioinks—specialized materials mixed with living cells—to build three-dimensional structures that mimic human tissues. This layer-by-layer approach allows for the creation of intricate models that replicate the spatial and mechanical properties of natural organs, a feat unattainable by conventional two-dimensional methods. The technology builds on advancements in traditional 3D printing and biomaterials science, adapting these principles to handle delicate biological components with unprecedented accuracy.
The emergence of this tool marks a pivotal moment in tissue engineering and regenerative medicine. It addresses critical gaps in research by providing platforms that closely simulate the human body’s environment, thus enhancing the study of disease progression and therapeutic responses. As a bridge between lab experiments and clinical applications, 3D bioprinting stands out in the broader technological landscape for its potential to revolutionize how complex health issues are approached.
Key Components and Operational Mechanisms
Bioinks: The Foundation of Tissue Fabrication
Central to 3D bioprinting are bioinks, which are hydrogel-based materials designed to act as scaffolds for cell growth, mimicking the extracellular matrix found in human tissues. These materials must balance biocompatibility with structural integrity, ensuring that cells remain viable while forming functional tissue-like constructs. Their composition often includes natural polymers like collagen or synthetic alternatives tailored to specific mechanical and biological needs.
The significance of bioinks lies in their ability to support cellular interactions and tissue development. Advanced formulations now aim to replicate the stiffness and elasticity of specific organs, such as breast tissue, enabling more accurate disease models. This focus on material science continues to drive improvements, with ongoing research targeting enhanced cell survival and long-term functionality in printed structures.
Printing Techniques and Hardware Capabilities
The technology employs several printing methods, each suited to distinct applications. Extrusion-based printing, for instance, pushes bioinks through nozzles to create continuous structures, ideal for larger tissue scaffolds. Inkjet printing, on the other hand, deposits tiny droplets for high-resolution patterns, while laser-assisted techniques offer precision for delicate cellular arrangements, catering to complex multi-cellular designs.
Hardware performance plays a crucial role in determining the success of these processes. Modern bioprinters are evaluated on precision, speed, and their capacity to handle diverse bioinks and cell types without compromising viability. The ability to fabricate intricate structures, such as microvascular networks, remains a key metric, with recent advancements pushing the boundaries of what these machines can achieve in terms of detail and scale.
Innovations and Current Trends
Recent progress in 3D bioprinting showcases remarkable strides in bioink development, with new formulations achieving greater fidelity to natural tissues. These improved materials better replicate the mechanical and biochemical cues of human environments, enhancing the realism of models used in research. Such advancements are critical for applications requiring precise simulations, like cancer studies.
A notable trend is the integration of artificial intelligence to optimize design and printing processes. AI algorithms now assist in predicting structural outcomes and refining tissue architecture, reducing trial-and-error in model creation. Additionally, high-throughput bioprinting systems are gaining traction for drug screening, allowing rapid production of multiple tissue samples for testing purposes.
Interdisciplinary collaboration further fuels innovation, as bioengineering merges with fields like molecular biology and materials science. Efforts toward microvascular printing, which aims to embed functional blood vessels within tissues, exemplify this synergy. These trends signal a shift toward more sophisticated and scalable solutions in the bioprinting domain.
Applications in Medicine and Research
One of the most impactful uses of 3D bioprinting lies in creating tissue models for disease research, particularly in oncology. Breast cancer tumor modeling, for instance, benefits immensely from bioprinted constructs that replicate the tumor microenvironment, including cellular interactions and mechanical properties. This enables deeper insights into cancer growth and metastasis under controlled conditions.
Beyond oncology, the pharmaceutical industry leverages this technology for drug testing, using bioprinted tissues to assess efficacy and toxicity before clinical trials. In regenerative medicine, research into organ transplantation explores the feasibility of printing functional tissues, addressing donor shortages. These applications highlight the versatility of bioprinting across medical sectors.
Unique use cases, such as personalized tumor models derived from patient cells, underscore the technology’s potential in precision medicine. By testing therapies outside the body, clinicians can identify optimal treatments tailored to individual profiles, minimizing the risks of ineffective interventions. This customization marks a significant leap forward in improving patient outcomes.
Challenges Hindering Widespread Adoption
Despite its promise, 3D bioprinting faces substantial technical barriers. Achieving full physiological accuracy in printed tissues remains elusive, particularly in replicating functional vascular networks essential for nutrient delivery. Long-term viability of constructs also poses a hurdle, as maintaining cell health over extended periods requires further material and design breakthroughs.
Regulatory landscapes present additional obstacles, with the lack of standardized protocols complicating clinical approvals. Ensuring safety and efficacy for patient use demands rigorous frameworks, which are still under development. These gaps highlight the need for cohesive guidelines to facilitate translation from research to real-world application.
Market challenges, including high costs and scalability issues, further limit accessibility. The expense of advanced bioprinters and specialized bioinks restricts adoption to well-funded institutions, while producing tissues at scale for widespread use remains impractical. Current efforts focus on developing cost-effective materials and streamlined processes to overcome these economic barriers.
Future Trajectory and Potential Impact
Looking ahead, 3D bioprinting holds the promise of printing fully functional organs for transplantation, a breakthrough that could redefine treatment for countless conditions. Research from 2025 onward aims to refine vascularization techniques and bioink durability, targeting viable solutions within the next few years. Such advancements could dramatically alleviate organ shortages globally.
The technology’s role in precision medicine also appears poised for expansion. Routine testing outside the body using patient-specific models could become standard, enabling therapies fine-tuned to unique genetic and disease profiles. This shift would enhance treatment accuracy while reducing adverse effects, reshaping clinical decision-making.
Integration with fields like genomics and AI offers further transformative potential. Combining bioprinted models with molecular data could uncover novel therapeutic targets, while AI-driven simulations might predict outcomes with unparalleled precision. These convergences suggest that 3D bioprinting could become a cornerstone of future healthcare strategies.
Final Reflections and Next Steps
Reflecting on this exploration, 3D bioprinting emerges as a transformative force in medical research, delivering unparalleled accuracy in tissue modeling and paving the way for personalized therapies. Its capacity to replicate complex human environments stands out as a game-changer, while ethical benefits like reduced animal testing underscore its societal value. The journey reveals both remarkable achievements and persistent hurdles that shape its current standing.
Moving forward, stakeholders should prioritize collaborative research to address vascularization and scalability challenges, accelerating the path to clinical integration. Investment in accessible biomaterials and streamlined regulatory frameworks proves essential to democratize access. By fostering partnerships across bioengineering and technology sectors, the groundwork is laid for bioprinting to redefine organ regeneration and disease treatment in the years ahead.