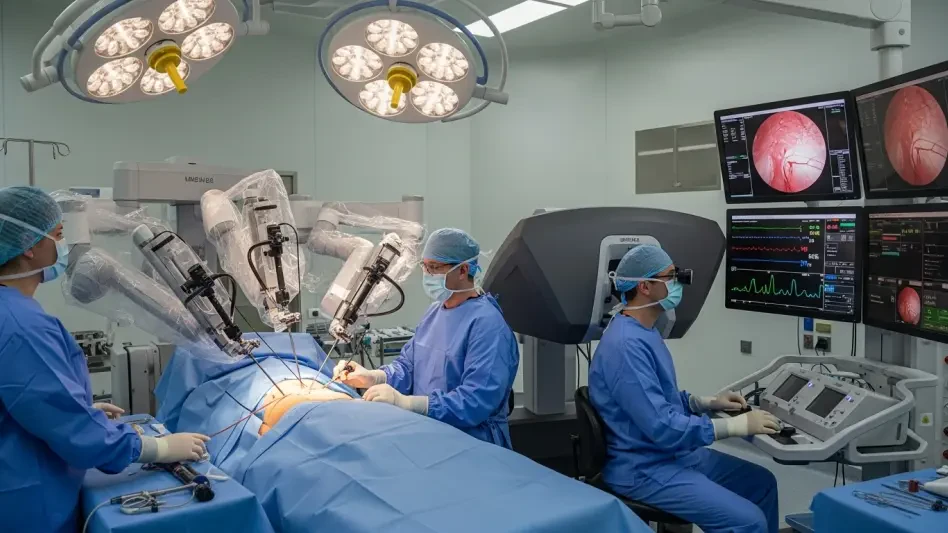
The Current Landscape of Vascular Surgery and Endovascular Robotics
Vascular surgery, particularly peripheral endovascular procedures, stands as a cornerstone of modern healthcare, addressing critical conditions like arterial blockages and aneurysms that affect millions annually. These minimally invasive interventions have become essential in reducing patient recovery times and improving outcomes compared to traditional open surgeries. With the U.S. alone witnessing approximately 2.5 million such procedures each year, the demand for precision and efficiency in this field continues to escalate, driven by an aging population and rising incidences of cardiovascular diseases.
The integration of robotics into vascular interventions has marked a significant shift, offering enhanced control and reducing risks for both patients and physicians. Major players like Microbot Medical and Siemens Healthineers have been at the forefront, leveraging technological advancements to redefine procedural standards. Robotic systems provide unparalleled accuracy in navigating complex vascular pathways, minimizing human error, and opening new possibilities for treating previously challenging cases.
This market’s growth is underscored by a pressing need for innovative solutions that can keep pace with the volume of procedures. The substantial scale of the U.S. market reflects not only the current burden on healthcare systems but also the opportunity for transformative tools to alleviate physician strain and elevate patient care. As technology evolves, the focus remains on balancing cutting-edge capabilities with accessibility to ensure widespread adoption across diverse medical facilities.
Unveiling the Liberty Endovascular Robotic System
Innovative Features and Clinical Advantages
The Liberty Endovascular Robotic System by Microbot Medical introduces a groundbreaking approach to peripheral vascular interventions with its single-use design and remote operation capabilities. Unlike traditional setups, this system allows physicians to control instruments via a video game-style controller, enabling precise navigation through blood vessels from a safe distance. Such a design not only enhances maneuverability but also streamlines the procedural setup, making it adaptable to various clinical environments.
Clinical studies conducted across multiple U.S. medical centers have demonstrated remarkable benefits of the Liberty system. A striking 92% reduction in radiation exposure for physicians addresses a long-standing occupational hazard, while a 100% success rate in robotic navigation to targeted sites highlights its reliability. Additionally, the absence of device-related adverse events in these trials reinforces the system’s safety profile, positioning it as a promising tool for enhancing procedural outcomes.
These advantages translate into tangible improvements for healthcare delivery, reducing physical strain on medical professionals and potentially shortening procedure times. By prioritizing both physician well-being and patient safety, the system sets a new benchmark for what robotic assistance can achieve in vascular surgery. Its innovative features could pave the way for broader acceptance of such technologies in routine clinical practice.
Market Positioning and FDA Clearance Impact
Achieving FDA 510(k) clearance represents a pivotal milestone for Microbot Medical, granting access to the expansive U.S. market for peripheral vascular procedures. This regulatory approval validates the Liberty system’s design and performance, providing a competitive edge as the company prepares for commercial rollout. The clearance serves as a gateway to address the needs of millions of patients while establishing credibility among healthcare providers.
A key differentiator for the Liberty system lies in its cost-effective framework, which eliminates the need for dedicated cath-lab rooms and specialized staff. This approach reduces financial burdens on hospitals and clinics, making the technology more accessible compared to other robotic systems that require significant infrastructure investments. Such affordability could accelerate adoption, particularly in facilities with constrained budgets seeking advanced solutions.
Positioning itself as an alternative to existing platforms, the system targets a niche where cost and ease of integration are paramount. By focusing on peripheral interventions, Microbot Medical taps into a segment with substantial demand but limited robotic solutions. This strategic alignment, bolstered by regulatory success, enhances the company’s potential to carve out a significant market share in the evolving landscape of endovascular robotics.
Challenges in Adopting Robotic Systems in Vascular Surgery
Despite the promise of robotic systems like Liberty, adoption in vascular surgery faces several hurdles that could slow widespread implementation. Financial constraints remain a primary barrier, as many healthcare facilities grapple with tight budgets that limit investments in new technologies, even those with long-term cost-saving potential. The initial costs of acquiring and maintaining such systems often deter smaller institutions from embracing innovation.
Beyond economics, the need for specialized physician training poses another challenge, as integrating robotics into existing workflows requires time and resources. Clinicians must adapt to new operational paradigms, which can disrupt established practices and demand ongoing support. Ensuring seamless incorporation into daily routines without compromising patient care adds a layer of complexity to the adoption process.
Microbot Medical also navigates internal challenges, with cash reserves of $4.1 million as of mid-year, supplemented by a $630,000 grant, highlighting the urgency of an efficient commercialization strategy. To overcome these obstacles, strategic partnerships with larger entities and scalable manufacturing processes could provide the necessary support. Addressing these barriers through collaboration and innovation will be critical to realizing the full potential of robotic systems in this field.
Regulatory Landscape and Compliance in Medical Robotics
The FDA 510(k) clearance for the Liberty system underscores the stringent safety and efficacy standards that medical robotics must meet to gain market entry. This regulatory milestone confirms that the technology adheres to rigorous benchmarks, ensuring reliability for clinical use. Such oversight is vital in maintaining trust among healthcare providers and patients as robotic interventions become more prevalent.
Broader trends in medical robotics regulation reveal an evolving framework that demands continuous compliance as technologies advance. Companies must anticipate potential future requirements, such as enhanced data reporting or post-market surveillance, to stay ahead of policy shifts. Navigating these expectations is particularly challenging for smaller firms like Microbot Medical, which lack the extensive resources of larger competitors.
Regulatory landscapes also shape market dynamics by influencing competition and entry barriers. For emerging players, achieving and maintaining compliance can be a significant differentiator, offering a pathway to establish credibility against established giants. As standards evolve, aligning innovation with regulatory demands will remain a cornerstone of success in this highly scrutinized sector.
Future Prospects for Liberty and Endovascular Robotics
Looking ahead, the Liberty system holds the potential to redefine vascular surgery by alleviating physician fatigue and enhancing patient outcomes through precise, remote-controlled interventions. Its focus on reducing occupational hazards like radiation exposure aligns with broader industry goals of improving workplace safety. If successfully commercialized, this technology could become a standard in peripheral procedures, setting a precedent for future innovations.
Industry trends indicate a move toward specialization, as seen with Siemens Healthineers pivoting to neurovascular interventions, leaving gaps in peripheral vascular segments that Microbot Medical is well-positioned to fill. This shift creates opportunities for smaller companies to address underserved areas, provided they can navigate competitive pressures from emerging players like Robocath. Capitalizing on these niches could solidify Liberty’s role in the market over the coming years, particularly from now through 2027.
Global expansion also presents a viable growth avenue, with untapped markets eager for cost-effective robotic solutions. However, economic conditions and innovation cycles will shape the trajectory of endovascular robotics, influencing adoption rates and investment levels. Monitoring these factors, alongside competitor movements, will be essential for stakeholders aiming to predict the long-term impact of systems like Liberty on a worldwide scale.
Conclusion: Redefining Vascular Surgery with Liberty Robotic System
Reflecting on the insights gathered, it becomes evident that the Liberty Robotic System carries transformative potential for vascular surgery through its clinical benefits, cost-efficiency, and timely FDA clearance. The system’s ability to reduce radiation exposure and ensure procedural success stands out as a game-changer for physician safety and patient care. Its strategic market positioning further highlights an opportunity to address critical gaps in peripheral interventions.
For stakeholders, the next steps involve closely tracking Microbot Medical’s commercialization efforts to gauge the system’s real-world impact. Exploring partnerships and funding opportunities emerges as a vital strategy to bolster scalability and market penetration. Additionally, investing in physician training programs is seen as a necessary measure to facilitate smoother integration into clinical settings.
Looking back, the broader implications for vascular surgery suggest that Liberty has the capacity to establish new benchmarks for safety, accessibility, and innovation. As the field continues to evolve, maintaining a focus on regulatory compliance and global outreach is deemed crucial for sustaining momentum. These actionable considerations provide a roadmap for navigating the challenges ahead and maximizing the system’s contributions to healthcare.